Chirothrips manicatus Haliday, 1836
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, pedicel and segments III-V with simple sense cone, terminal segments V-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore wing and fore wing distal region
Fig. 6: Hind coxae and first sternite
Fig. 7: Sternites III and IV
Fig. 8: Sternites VI and VII
Fig. 9: Tergites V and VI
Fig. 10: Tergites VIII-XI
Introduction and recognition
Chirothrips manicatus is a widely reported pest of grasses, particularly when grown for seed production. Female macropterous; body, antennae and legs brown, tarsi paler; fore wings light brown. Antennae 8-segmented; segment II with prolonged external margin and bearing an exactly terminal small seta-like sensorium at tip, III & IV each with stout simple sense cone (Fig. 1). Head small and prolonged in front of eyes; with 3 pairs of ocellar setae, pair III anterolateral to fore ocellus (Fig. 2). Pronotum trapezoidal; with 2 pairs of elongate posteroangular setae (Fig. 3). Meso- and metafurca with well-developed lateral flanges, but without median spinula. Metanotum reticulate; median pair of setae shorter than lateral pair and arise behind anterior margin; campaniform sensilla present (Fig. 4). Mid and hind tarsi 2-segmented; fore tibia not prolonged around fore tarsus. Fore and hind wing present, more than half as long as abdomen (macropterous) or micropterous, fore wing pointed at apex; first vein with 2 setae on distal half; second vein with 4 setae (Fig. 5). Tergites with antecostal ridge strong and complete lines of sculpture medially; posterior margin of tergites II-VIII with continuous and weakly rounded lobed craspedum; ovipositor weak with faint teeth (Fig. 9 and 10). Sternites with 3 pairs of marginal setae, median pair on VII arise in front of margin; posterior margin of sternites II-V with craspedum of distinctive tubercles, also laterally on VI but not on VII (Fig. 7 and 8).
Male micropterous; tergites with strong lines of sculpture medially; sternites III-VII with small circular glandular area.
Taxonomic identity
Species
Chirothrips manicatus Haliday, 1836
Taxonomic history
Chirothrips longisetis Priesner, 1949
Chirothrips bagnalli Hood, 1938
Chirothrips testacea Hukkinen, 1935
Chirothrips ambulans Bagnall, 1932
Chirothrips productus Bagnall, 1932
Chirothrips laingi Bagnall, 1932
Chirothrips takahashii Moulton, 1928
Chirothrips ammophilae Bagnall, 1927
Chirothrips albicornis Priesner, 1926
Chirothrips microptera Maltbaek, 1921
Chirothrips brachyptera Maltbaek, 1921
Chirothrips aptera Schille, 1911
Chirothrips similis Bagnall, 1909
Chirothrips fusca Coesfeld, 1898
Chirothrips adusta Uzel, 1895
Chirothrips antennatus Osborn, 1883
Thrips longipennis Burmeister, 1838
Thrips (Chirothrips) manicatus Haliday, 1836
Common name
Timothy thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Chirothrips Haliday, 1836
Genus description
The genus Chirothrips Haliday, 1836
Chirothrips currently includes about 45 to 50 species worldwide. Zur Strassen (1960) provided an identification key and catalogue of this genus for over 50 species, and Bhatti (1990) created six new genera for these species originally placed in the genus Chirothrips, and one of these, Arorathrips, is discussed in comparison to Chirothrips. Chirothrips appears to be a genus of Holarctic species, whereas Arorathrips is from the New World with a typical reduced mesothoracic endofurca. This genus has consistent characters that include small heads, short antennae with 8 segments, antennal segment II mostly expanded laterally (except in this key for Chirothrips guillarmodi), and a trapezoidal pronotum with 2 pairs of long posteroangular setae.
Species description
Typical key character states of Chirothrips manicatus
Coloration and body sculpture
Body color: mainly brown to dark brown
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Form of sense cones on antennal segments III and IV: emergent and simple on segments III and IV
Number of antennal segments: 8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: asymmetric with prolonged external margin bearing a terminal small seta at tip
Antennal segment III shape: symmetric
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: distinctly prolonged in front of compound eyes
Ocellar setae I: present
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle
Ocelli: present
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 3
Prothorax
Number of pairs of long anteroangular setae: 0
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 2
Pronotal blotch or internal apodeme: absent
Pronotum shape: trapezoidal
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: without spinula
Metathorax
Metanotal campaniform sensilla: present
Metanotal median setae: S1 behind anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: shorter than lateral metanotal setae
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous) or absent (apterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing number of setae of second vein: 4-6
Fore wing second vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fore wing first vein number of setae on distal half: 2
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: dark
Fore wings: uniformly light brown
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Abdomen
Pleurotergites: not covered in microtrichia
Sternite II: with marginal setae but no discal setae
Craspedum on sternites II to V: present, craspedum with entire margin and a series of distinctive tubercles
Sternites IV, V and VI: with marginal setae but no discal setae
Sternite VII median posteromarginal setae S1: arising in front of posterior margin
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Tergites II to VIII discal campaniform sensilla: situated in front of the discal setae S1 but somewhat farther apart than the setae
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Tergites IV and V median setal pair: shorter than distance between their bases
Tergites V to VII: without ctenidia laterally, but sometimes with rows of microtrichia
Craspedum on tergites IV to VI: present, continuous craspedum with margin of weakly and rounded lobes
Tergite VIII ctenidia: without paired ctenidia laterally, sometimes with irregular microtrichia
Tergite VIII posteromarginal comb of microtrichia: absent
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
The species can be distinguished from other species of genus Chirothrips by the tergites bearing a continuous craspedum with margin of weakly and rounded lobes (in Chirothrips frontalis the tergites have a complete craspedum of unbroken border or flange at the posterior margin; Chirothrips ah and Chirothrips meridionalis with broadly rounded independent lobes fused at bases into continuous craspedum; Chirothrips guillarmodi with closely approximated but independent elongate lobes; and Chirothrips pretorianus has a craspedum with independent small, triangular lobes), and the posterior margin of sternites is entire with a series of distinctive tubercles (Chirothrips frontalis and Chirothrips pretorianus without sternal craspedum; Chirothrips ah and Chirothrips meridionalis with a craspedum of independent small pointed lobes; and Chirothrips guillarmodi with closely approximated but independent elongate lobes). Chirothrips manicatus as well as Chirothrips frontalis have a asymmetric antennal segment II with prolonged external margin bearing an exactly terminal small seta at tip. Whereas Chirothrips ah has a very slender and acute, slightly hook-shaped projection of the second antennal segment which bears a small sensorium at its apex, in Chirothrips guillarmodi antennal segment II is symmetric, in Chirothrips meridionalis it is not strongly produced on outer margin, but drawn out into a sharply pointed angle, and in Chirothrips pretorianus it is asymmetric with prolonged external margin bearing a apical sense area. Chirothrips manicatus and most of Chirothrips species with ocellar setae III arranged anterolateral to fore ocellus, and metanotal median setae are shorter than the lateral pair and arising behind anterior margin (except for Chirothrips ah with ocellar setae III on anterior margins of ocellar triangle and median metanotal setae which are distinctly longer than lateral metanotal setae and arising at anterior margin; Chirothrips guillarmodi and Chirothrips meridionalis with metanotal median setae arising at anterior margin). Tergites II-VIII discal campaniform sensilla situated in front of the discal setae S1 but somewhat farther apart than the setae (as well as in Chirothrips pretorianus and Chirothrips frontalis). Whereas Chirothrips ah, Chirothrips guillarmodi and Chirothrips meridionalis have tergites II-VIII discal campaniform sensilla placed between the discal setae S1 and S2 or posteriorly.
Species of the genus Chirothrips are similar to Arorathrips mexicanus, but Arorathrips mexicanus has a greatly reduced mesothoracic furca (furcal pits widely separated), fore tibia prolonged at apex around the external margin of the first fore tarsal segment, and tergites II-V with transverse row of small tubercles along antecostal ridge. Whereas all species of the genus Chirothrips have furcal pits on mesosternum fused in the middle line, fore tibia not prolonged around fore tarsal segment, and the sculpture of antecostal ridge on tergites II-V is more or less elongated. Compared to described species of Chirothrips, Arorathrips mexicanus has the sternite VII median posteromarginal setae arising at margin, whereas in species of Chirothrips these setae arise in front of posterior margin.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The full cycle can take less than a week to over a month and adults may live for more than one month producing several generations in one year depending on seasonal weather (Lewis 1973).
Host plants
Various Poaceae species including cereal crops, with no recorded specificity and weed plants like Tagetes minuta and Tephrosia villosa ssp. eherenbergiana.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
All species in this genus Chirothrips breeding in grass flowers, sometimes their larvae and especially pupae are transported around the world within commercial grass seed.
Detection and control strategies
-
Additional notes
The precise host-plant on which this species breeds is not clear, but like all members of the genus Chirothrips the females lay eggs in the young flowers of certain grasses. Each larva apparently feeds within, and subsequently pupates within a single floret.
Biogeography
World wide temperate regions, Africa, Asia, Australia, New Zealand, North America, Central and South America, Europe. Kenya, Morocco (Marrakech),
Tunisia (Ferme Shitta - Djebel Eddyr / 1100m).
African countries where Chirothrips manicatus has been reported
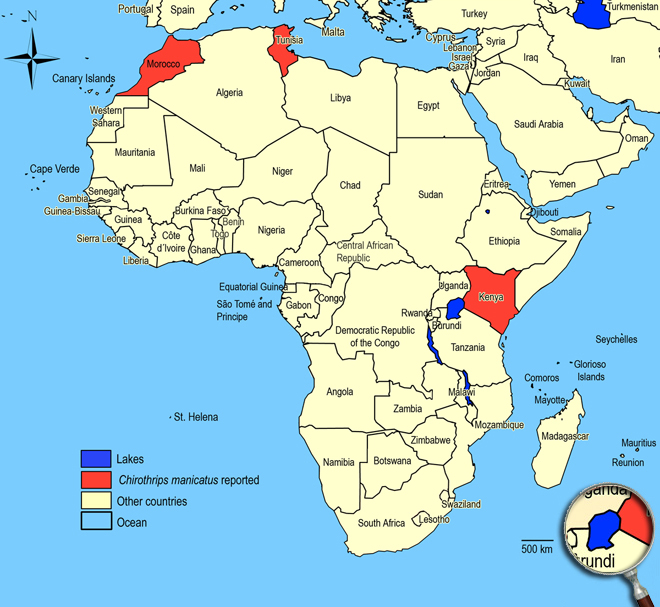
Occurence of Chirothrips manicatus in East Africa
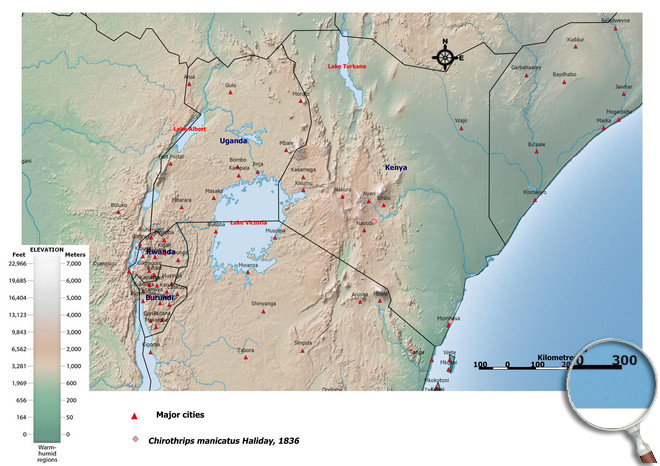
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Chirothrips manicatus in parts of East Africa.

Bibliography
Bagnall RS (1909). A contribution to our knowledge of the British Thysanoptera (Terebrantia), with notes on injurious species. Journal of Economic Biology. 4: 33-41
Bagnall RS (1927). Contributions towards a knowledge of the European Thysanoptera. II. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 19: 564-575
Bagnall RS (1927). Contributions towards a knowledge of the European Thysanoptera. III. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 20: 561-585
Bagnall RS (1932). Preliminary descriptions of some new species of Chirothrips (Thysanoptera). Entomologist's Monthly Magazine. 68: 183-187
Bailey SF (1957). The thrips of California. Part I: Suborder Terebrantia. Bulletin of the California Insect Survey. 4 (5): 143-220
Bhatti JS (1990). On some genera related to Chirothrips (Insecta: Terebrantia: Thripidae). Zoology (Journal of Pure and Applied Zoology). 2 (4): 193-200
Burmeister H (1838). Handbuch der Entomologie, Vol. 2 (2), pp. 397-1050. Verlag Enslin, Berlin
Engel R & Ohnesorge B (1994). The role of alternative food and microclimate in the system Typhlodromus pyri Scheuten (Acari, Phytoseiidae) - Panonychus ulmi Koch (Acari, Tetranychidae) on grape vines. 1. Laboratory investigations. Journal of Applied Entomology. 118 (2): 129-150
Engel R & Ohnesorge B (1994). The role of alternative food and microclimate in the system Typhlodromus pyri (Acari, Phytoseiidae) - Panonychus ulmi (Acari, Tetranychidae) on grape vines. 2. Field experiments. Journal of Applied Entomology. 118 (3): 224-238
Haliday AH (1836). An epitome of the British genera, in the order Thysanoptera, with indications of a few of the species. The Entomological Magazine. 3 (5): 439-451
Hood JD (1938). On some European species of Chirothrips (Thysanoptera). Entomologist's Monthly Magazine. 74: 158-164
Ishii J & Kadono Y (2002). Factors influencing seed production of Phragmites australis. Aquatic Botany. 72 (2): 129-141
Jenser G (1982). Data to the Thysanoptera fauna of Tunisia. Folia Entomologica Hungarica. 43 (1): 55-57
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp.
Minaei K & Mound LA (2010). Grass-flower thrips of the genus Chirothrips (Thysanoptera: Thripidae), with a key to species from Iran. Zootaxa. 2411: 33-43
Morison GD (1928). Observations and records for some Thysanoptera from Great Britain III. Chirothrips manicatus Hal. and Limothrips spp. Entomologist’s Monthly Magazine. 64: 189-196
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1911). Synopsis, catalogue and bibliography of North American Thysanoptera, with descriptions of new species. Technical Series, USDA, Bureau of Entomology. 21: 1-56
Mound LA (1968). A review of R. S. Bagnalľs Thysanoptera collections. Bulletin of the British Museum (Natural History), Entomology. Supplement 11: 1-181
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainesville, 487 pp
Mound LA, Morrison GD, Pitkin BR & Palmer JM (1976). Thysanoptera. Handbooks for the identification of British insects, Vol. 1, Part 11. Royal Entomological Society of London, London, 79 pp
Mound LA & Palmer JM (1972). Grass-flower infesting thrips of the genus Chirothrips Haliday in Australia. Journal of the Australian Entomological Society. 11: 332-339
Nakahara S & Footit RG (2012). Review of Chirothrips and related genera (Thysanoptera: Thripidae) of the Americas, with descriptions of one new genus and four new species. Zootaxa 3251: 1-29
North RC & Shelton AM (1986). Overwintering of the onion thrips, Thrips tabaci (Thysanoptera, Thripidae), in New York. Environmental Entomology. 15 (3): 695-699
Osborn H (1883). Notes on Thripidae, with descriptions of new species. Canadian Entomologist. 15 (8): 151-156
Pelikán J (1988). Records, notes and list of Thysanoptera from Algeria. Acta Entomologica Bohemoslovaca. 85: 21-27
Pelikán J, Fedor P, Krumpal M & Cyprich D (2002). Thrips (Thysanoptera) in nests of birds and mammals in Slovakia. Ekologia, Bratislava. 21 (3): 275-282
Pitkin BR (1972). So-called spermatophores of Chirothrips manicatus Haliday (Thysanoptera-Thripidae). Journal of Entomology, Series A, General Entomology. 46: 1-149
Priesner H (1925). Thysanopterologica I. Zoologisches Jahrbücher. 50: 305-319
Priesner H (1926-28). Die Thysanopteren Europas. F. Wagner Verlag, Wien, 755 pp
Priesner H (1949). Studies on the genus Chirothrips Hal. (Thysanoptera). Bulletin de la Société Fouad I ďEntomologie. 33: 159-174
Roditakis E & Roditakis NE (2007). Assessment of the damage potential of three thrips species on white variety table grapes - In vitro experiments. Crop Protection. 26 (4): 476-483
Stannard LJ (1968). The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 214-552
Uzel H (1895). Monographie der Ordnung Thysanoptera. Uzel, Königgrätz, 473 pp.
zur Strassen R (1958). Studies in African Thysanoptera, 1. Journal of the Entomological Society of Southern Africa. 21 (2): 333-353
zur Strassen R (1959). Eight synonyms amongst the European species of Chirothrips Haliday, 1836 (Thysanoptera). Journal of the Entomological Society of Southern Africa. 22 (1): 88-107
zur Strassen R (1960). Key to and catalogue of the known species of Chirothrips Haliday, 1836 (Thysanoptera: Thripidae). Journal of the Entomological Society of Southern Africa. 23 (1): 144-176
zur Strassen R (1974). Weitere Thysanopteren-Arten aus Marokko (Ins.: Thysanoptera). Senckenbergiana Biologica. 55 (1-3): 135-139
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California














